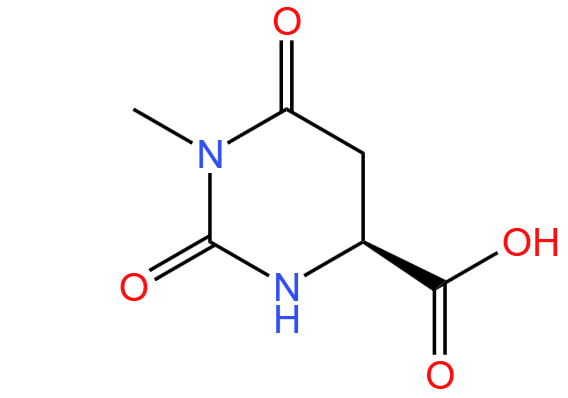
1-Methyl-L-4,5-dihydroorotic acid
CAS NO:103365-69-1
GMP Commercial Production
Our company's commercial production project, GMP system
High Quality and High Purity
With multiple iterations of technology, our product quality has consistently surpassed industry standards, and we are able to control multiple variables based on different customer needs.
In stock
Regularly stocked items, available in stock for quick shipment
Contact our consultant to obtain the COA report
Product Usage
Specification
This product serves as an intermediate for Taltirelin, a drug developed by Mitsubishi Tanabe Pharma Corporation in Japan. It was launched in Japan in 2000 for improving ataxia in patients with spinocerebellar ataxia. The orally disintegrating tablets of Taltirelin, which are available in Japan, exhibit not only endocrine effects but also exert certain central nervous system (CNS) effects.
Molecular mass
172.14
Molecular formula
C6H8N2O4
Appearance
White powder
Purity
≥99%
Product Usage
This product serves as an intermediate for Taltirelin, a drug developed by Mitsubishi Tanabe Pharma Corporation in Japan. It was launched in Japan in 2000 for improving ataxia in patients with spinocerebellar ataxia. The orally disintegrating tablets of Taltirelin, which are available in Japan, exhibit not only endocrine effects but also exert certain central nervous system (CNS) effects.
Specification
Molecular mass
172.14
Molecular formula
C6H8N2O4
Appearance
White powder
Purity
≥99%

Contact us
Manager Wang:(+86)18321962163
Manager Lin:(+86)13564545227
Email: sales@eyougene.com

Scan the QR code and follow us
Follow our WeChat official account
Shanghai Eyougene Biotechnology Development CO.,LTD.

Contact us
Manager Wang:(+86)18321962163
Email: sales@eyougene.com

Scan the QR code and follow us
Follow our WeChat official account
Shanghai Eyougene Biotechnology Development CO.,LTD.
Advanced Products
About Eyougene
Shanghai Eyougene Co., Ltd. is a next-generation digital enterprise based on its own leading chemical technology, focusing on the fields of pharmaceuticals and new materials. We are committed to providing one-stop solutions for customers in the fields of innovative drugs and new materials both domestically and internationally.
Since its establishment in 2015, Eyougene has experienced rapid development, steadily achieving integration from market, research and development to manufacturing facilities, possessing integrated operational capabilities from research and development to commercialization. The company currently has three research centers located in core cities such as Shanghai, Xi'an, and Wuhan, equipped with advanced reaction equipment and detection instruments including Lauda temperature control, fixed bed reactors, pressure reactors, HPLC-MS, and more. Our GMP & FDA dual-certified factory is located in the picturesque and conveniently located Jiande, Hangzhou, built entirely according to GMP standards and utilizing DCS automated control management. It is capable of conducting various special reactions and high-standard experiments. This GMP factory has passed audits from several top global companies, and its system capabilities have been widely recognized.
Eyougene's founding team and core members come from top universities and research institutes both domestically and abroad. With years of accumulation, we have incubated several distinctive technology platforms and developed a rich product pipeline. Our company has earned high-quality business from domestic and international leading enterprises and received wide acclaim from customers due to our high-level product quality and competitive cost advantages.
Core Technology
01
02
03
04
Inside Eyougene
R&D Center
GMP Factory
Partners

















